Understanding PCOD: Evidence-Based Insights on Hormonal Imbalance, Metabolic Risks, and Treatment Approaches- A comprehensive scientific review.
Dr JK Avhad MBBS MD [Last updated 28.12.2025]
Polycystic Ovarian Disease (PCOD),
often used interchangeably with Polycystic Ovary Syndrome (PCOS), is one of the
most common endocrine–metabolic disorders affecting women of reproductive age.
Worldwide, PCOD/PCOS affects 8–20%
of women, though prevalence varies widely depending on diagnostic criteria,
ethnicity, and lifestyle patterns. It is not only a reproductive disorder but
also a systemic metabolic condition involving insulin resistance, chronic
low-grade inflammation, dyslipidemia, and increased long-term cardiometabolic
risk.
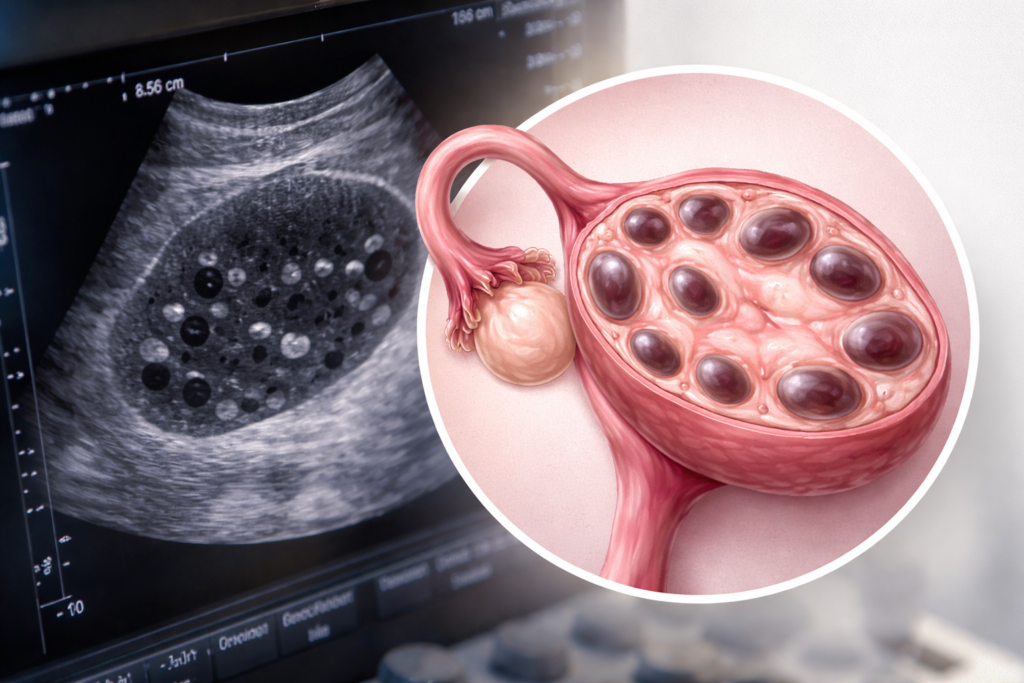
PCOD is characterized by ovarian dysfunction, multiple immature follicles in the ovaries,
and hormonal imbalance,
especially excess androgen production. Unlike PCOS, which is defined by a set
of criteria (like Rotterdam, NIH, AE-PCOS), PCOD is often used in India and South Asia to describe ovarian cystic
changes dominated by lifestyle driven hormonal dysregulation rather than
classic syndrome-level metabolic involvement.
Although the terms overlap, modern
research leans toward the unified name PCOS, since the ovarian cysts
themselves are not the central issue, the endocrine disruption is. Still, many
clinicians differentiate them as:
- PCOD:
A milder, reversible ovarian condition with irregular ovulation and cyst
formation.
- PCOS:
A broader syndrome with metabolic, hormonal, and reproductive
consequences.
Regardless of terminology, the
underlying pathophysiology presents significant implications for menstrual
health, fertility, long-term metabolic health, and quality of life.
Epidemiology and Global Trends
PCOD/PCOS prevalence varies across
regions:
- India:
Estimates range from 12–36% depending on urbanization, diet, and
diagnostic standards.
- Global average:
10–15% among reproductive aged women.
- Adolescents:
Increasing incidence due to earlier puberty, sedentary lifestyle, and
rising obesity rates.
There is a clear trend toward younger
age of onset, often starting soon after menarche, and increasingly linked
to modern diet and lifestyle patterns.
Factors associated with higher
prevalence:
- Urban life
- High BMI
- Sedentary lifestyle
- High sugar and processed food consumption
- Genetic predisposition [ family history of diabetes or
PCOS ]
Pathophysiology of PCOD
PCOD arises from complex interactions between genetic factors, hormones, metabolism, and environmental factors. No single cause explains the disorder, instead, it is a cluster of interacting abnormalities.
Insulin Resistance: The Central culprit
Insulin resistance [ IR ] is found in 50–70% of women with PCOD/PCOS, even in those with normal body weight. IR triggers a cascade of hormonal events:
- Elevated insulin → stimulates ovarian theca cells → increased androgen production.
- Insulin inhibits hepatic production of sex hormone binding globulin (SHBG) → increasing free testosterone.
- IR contributes to abdominal fat deposition → worsening inflammation and hormonal imbalance.
Hyperandrogenism
Excess androgens [ testosterone, androstenedione, DHEA-S ] lead to:
- Hirsutism (Excessive facial, body hair)
- Acne
- Scalp hair thinning (androgenic alopecia)
- Ovulatory dysfunction
The ovaries overstimulated by LH and insulin produce more androgens than normal.
Ovarian Dysfunction and Follicular Arrest
Normally, one follicle matures each cycle. In PCOD:
- Follicles start developing → but do not mature → they accumulate.
- Ovaries enlarge with multiple small follicles (2–9 mm).
- The chronic anovulation leads to progesterone deficiency and unopposed estrogen dominance.
Chronic Low-Grade Inflammation
Women with PCOD often show elevated inflammatory markers (CRP, TNF-α, IL-6). Chronic inflammation contributes to:
- Insulin resistance
- Endothelial dysfunction [ Endothelium is inner lining of your blood vessels ]
- Increased cardiovascular risk
Genetic Factors
Variants in genes related to insulin signaling, androgen production, and gonadotropin action have been linked to PCOD. Family clustering is common, particularly with type 2 diabetes, obesity, or metabolic syndrome.
Clinical Features
PCOD manifestations vary widely. Some women have mild symptoms; others experience a full spectrum of reproductive, metabolic, and psychological issues.
Menstrual Irregularities
- Oligomenorrhea
- Hypomenorrhea
- Amenorrhea (no periods for 3–6 months)
- Heavy bleeding due to endometrial buildup
- Difficulty predicting cycles
Hyperandrogenic Symptoms
- Moderate to severe acne
- Increased facial and body hair
- Hair thinning on scalp
- Darkening of skin
Weight Issues
- Tendency for central obesity
- Difficulty losing weight
- Water retention and bloating
Fertility Challenges
PCOD is one of the leading causes of female infertility due to anovulation.
Metabolic Symptoms
- Sugar cravings
- Fatigue
- Rapid weight gain
- Elevated cholesterol and triglycerides
Psychological Impact
Women frequently experience:
- Anxiety
- Depression
- Low self-esteem
- Body image issues.
Diagnostic Criteria
Rotterdam Criteria
A diagnosis is made if 2 out of 3 are present:
- Oligo- or anovulation
- Clinical or biochemical hyperandrogenism
- Polycystic ovaries on ultrasound [ ≥12 follicles OR ovarian volume >10 mL ]
Other causes must be excluded [ thyroid disorders, hyperprolactinemia, CAH, and more ]
Ultrasound Findings
- Enlarged ovaries
- Multiple small peripheral follicles (“string of pearls”)
- Increased ovarian stroma
Blood Tests
- Elevated LH levels or high LH:FSH ratio
- High testosterone / DHEA-S
- Elevated fasting insulin
- Dyslipidemia: ↑ TG, ↓ HDL
- Increased AMH (anti-Müllerian hormone)
PCOD vs PCOS: A Clear Distinction
Many patients and even some clinicians use the terms interchangeably, but in practice:
|
Feature |
PCOD |
PCOS |
|
Primary nature |
Ovarian dysfunction |
Multi-system endocrine-metabolic syndrome |
|
Severity |
Mild–moderate |
Often moderate–severe |
|
Metabolic issues |
Less common |
Very common |
|
Insulin resistance |
Can occur |
Frequently present |
|
Long-term risks |
Lower |
Higher |
|
Reversibility |
Often lifestyle-correctable |
More chronic |
Clinically, the management strategies overlap, but understanding this distinction helps tailor treatment intensity.
Complications of PCOD
Reproductive Complications
- Infertility
- Pregnancy complications: miscarriage, gestational diabetes mellitus (GDM), pregnancy induced hypertension (PIH)
- Endometrial hyperplasia (due to chronic estrogen exposure)
Metabolic Complications
- Insulin resistance → type 2 diabetes
- Dyslipidemia
- Non-alcoholic fatty liver disease (NAFLD)
- Metabolic syndrome
Cardiovascular Risk
- Hypertension
- Atherosclerosis
- Increased lifetime risk of heart disease
Psychological Effects
- 3x higher prevalence of depression
- 5x higher prevalence of anxiety
- Eating disorders, especially binge eating
Management of PCOD
Management is personalized, focusing on symptom control, menstrual regularization, and long-term prevention of metabolic disease.
Lifestyle Modifications:
Dietary Interventions
Key dietary principles:
- Low glycemic index foods
- High fiber
- High protein, moderate healthy fats
- Avoid refined sugar, fried foods, ultra-processed foods
- Regular meal timing to regulate insulin levels
Recommended eating patterns:
- Mediterranean diet
- Anti-inflammatory diet
- Low-carbohydrate diet
- Plate method (50% veggies, 25% protein, 25% whole grains)
Foods to focus on:
- Whole grains, lentils, legumes
- Leafy greens, cruciferous vegetables
- Nuts, seeds, avocados
- Oily fish (omega-3s)
- Berries, citrus
- Lean proteins
Foods to limit:
- Sugary drinks
- Pastries and white flour
- Red and processed meats
- Packaged snacks
- High-salt foods
Weight Management
Even a 5–10% reduction in body weight improves:
- Ovulation
- Insulin sensitivity
- Hormonal levels
- Acne and hair fall
Exercise
Combination of:
- Strength training (improves insulin sensitivity)
- Cardio (fat loss)
- High-intensity interval training
- Yoga and Pilates (hormonal balance)
Minimum target: 150 minutes/week moderate exercise + strength training 3 days/week.
Medications
Metformin
- Improves insulin sensitivity
- Helps regulate periods
- Aids weight loss in some women
Oral Contraceptives
- Regulate menstrual cycle
- Reduce acne and hair growth
- Control androgen levels
Anti-Androgens
- Spironolactone
- Flutamide
- Used for hirsutism and acne
Fertility Medications
- Letrozole (first-line drug)
- Clomiphene
- Gonadotropins
Other Therapies
- Inositols (Myo-inositol + D-chiro inositol)
- Omega-3 supplements
- Vitamin D correction
- Chromium picolinate
PCOD and Fertility
PCOD is the most common cause of anovulatory infertility.
Mechanisms:
- Irregular ovulation
- Poor egg quality
- Hormonal imbalance
- Endometrial changes
Treatment approach:
- Lifestyle modification
- Ovulation induction (Letrozole preferred)
- Metformin for insulin-resistant cases
- IVF/ICSI for resistant or complex cases
Success rates improve dramatically when weight and metabolism are optimized.
Long-Term Health Risks
Even after reproductive symptoms settle, the metabolic risks persist.
Diabetes
PCOD increases lifetime risk of type 2 diabetes by 400–700%.
Hypertension & Dyslipidemia
Due to chronic insulin resistance and inflammation.
Cardiovascular Disease
Higher lifetime risk for:
- Heart attack
- Stroke
- Atherosclerosis
Endometrial Cancer
Chronic anovulation → unopposed estrogen → increased endometrial cell proliferation
Sleep Disorders
High prevalence of obstructive sleep apnea.
Monitoring is essential:
- Annual glucose testing
- Lipid profile
- BP monitoring
- Liver function tests
- Mental health screening
PCOD in Adolescents
Diagnosing PCOD in teens is challenging because:
- Irregular periods are normal in the first 1–2 years after menarche
- Acne is common
- Ovaries are naturally multicystic during puberty
Thus, strict criteria are needed to avoid over-diagnosis:
- Persistent irregular cycles >2 years
- Clear signs of hyperandrogenism
- Biochemical confirmation
Lifestyle intervention is preferred over medications initially.
PCOD and Mental Health
Psychological effects are often overlooked but can be significant:
- Reduced confidence due to acne, hirsutism, weight issues
- Anxiety about fertility
- Body image disturbances
- Emotional eating
- Depression linked to chronic inflammation and hormonal imbalance
Holistic care includes:
- Therapy
- Support groups
- Stress management techniques
- Yoga and mindfulness
- Meditation
Modern Research and Future Directions
Emerging areas of interest:
- Gut microbiome: Dysbiosis plays a major role in insulin resistance and inflammation.
- Genetic profiling: Identifying high-risk individuals early.
- Personalized medicine: Tailoring therapy based on metabolic phenotype.
- Novel drugs: GLP-1 receptor agonists (e.g., semaglutide) for weight loss.
- Advanced reproductive techniques: Improved IVF outcomes for PCOD patients.
Conclusion
PCOD is a complex, multifactorial condition affecting millions of women worldwide. While often misunderstood as a simple ovarian issue, PCOD is fundamentally tied to metabolic dysfunction, hormonal imbalance, and inflammatory pathways. It influences reproductive health, long-term metabolic risk, cardiovascular health, mental well-being, and quality of life.
However, early diagnosis, structured lifestyle changes, targeted medical treatment, and ongoing monitoring can significantly improve outcomes. In many cases, PCOD is highly manageable and even reversible, especially when lifestyle modification is prioritized.
A strong patient–clinician partnership, awareness of the condition, and individualized care remain key to effective long-term management.
This article is for informational purpose only and does not substitute for professional medical advise. For proper diagnosis and treatment seek the help of your healthcare provider.
References:
1. Azziz, R., et al. (2016). Epidemiology and pathogenesis of the polycystic ovary syndrome. Nature Reviews Endocrinology, 12(4), 217–231.
2. Barry, J. A., et al. (2014). Endometrial cancer risk in women with PCOS. Human Reproduction Update, 20(5), 748–758.
3. Burt Solorzano, C., & McCartney, C. R. (2010). HPO axis and PCOS pathophysiology. Endocrinology and Metabolism Clinics, 39(4), 633–642.
4. Carmina, E., et al. (2006). Hyperandrogenism clinical aspects. Journal of Clinical Endocrinology and Metabolism, 91(1), 2–6.
5. Day, F., et al. (2018). Genomic analysis identifies PCOS susceptibility loci. Nature Communications, 9, 5247.
6. Deswal, R., et al. (2020). Global prevalence of PCOS: A systematic review. Reproductive Biology and Endocrinology, 18, 70.
7. Dokras, A., et al. (2018). Mental health and quality of life in PCOS. Human Reproduction Update, 24(6), 709–725.
8. Dunaif, A. (2012). Insulin resistance in PCOS. Endocrine Reviews, 33(3), 981–1030.
9. González, F., et al. (2012). Chronic inflammation in PCOS. Journal of Clinical Endocrinology and Metabolism, 97(11), 4061–4069.
10. Goodman, N. F., et al. (2015). AACE guidelines for PCOS management. Endocrine Practice, 21(11), 1291–1300.
11. Ibáñez, L., et al. (2017). PCOS in adolescence. Nature Reviews Endocrinology, 13(4), 217–227.
12. Legro, R. S., et al. (2013). Diabetes risk in PCOS. Journal of Clinical Endocrinology and Metabolism, 98(12), 4646–4654.
13. Legro, R. S., et al. (2014). Letrozole vs clomiphene for infertility. New England Journal of Medicine, 371, 119–129.
14. Lizneva, D., et al. (2016). PCOS epidemiology classification. Human Reproduction Update, 22(3), 284–300.
15. Lord, J., et al. (2003). Metformin and ovulation: Meta-analysis. BMJ, 327, 951.
16. Saxena, P., et al. (2020). Prevalence of PCOS in India. International Journal of Reproductive Medicine, 2020.
17. Setji, T. L., et al. (2006). NAFLD in PCOS. Endocrine Practice, 12(3), 287–293.
18. Torres, P. J., et al. (2018). Gut microbiome and PCOS. PLoS ONE, 13(1), e0190037.
Unfer, V., et al. (2012). Myo-inositol effects on ovulation. International Journal of Endocrinology, 2012.
